When I first started working in labs over a decade ago, “lab automation” usually meant one of two things: an instrument that automated one step of research, or a sophisticated, bespoke system that only well-funded facilities could afford. Even with the rise of lab automation technologies over the last decade, to this day, biology is still very manual. Biologists still pipette thousands of samples, manually track plates, and move samples step-by-step between their instruments. If something goes wrong with an automated protocol overnight, we find out the next morning.
However, the meaning of lab automation is changing. Automation is no longer just about speeding up one task or bespoke hardware integration. It’s increasingly about collecting better data across various devices, quickly designing complex experiments, utilizing hardware with significantly better reliability, and scaling capabilities without burdensome integration work. As we move into 2026, lab automation is becoming more accessible and far more powerful for biologists—even long-established vendors are being pushed to evolve and compete in ways they previously didn’t have to.
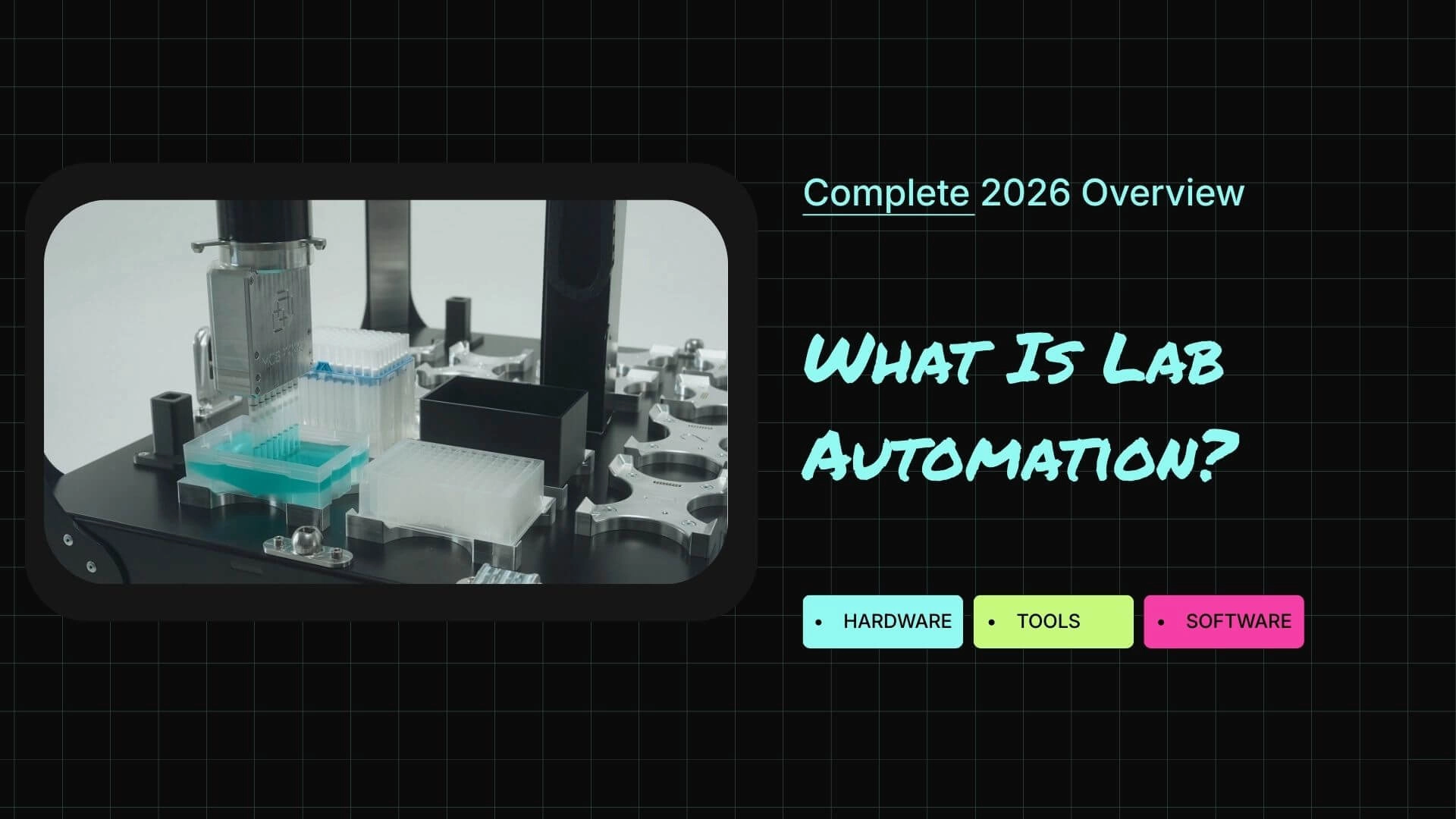
What is lab automation?
Lab automation is the use of automated hardware and software to perform laboratory tasks that would otherwise be done manually. This includes activities like liquid handling, sample preparation, plate movement, imaging, data capture, and experiment design.
At its core, lab automation exists to do five things well:
- Increase speed and throughput, allowing labs to run more experiments in less time
- Execute laboratory workflows consistently while reducing human error in repetitive tasks
- Enable experiments that can’t realistically be performed by hand, for example, miniaturization
- Extend effective lab capacity through overnight and unattended runs
- Free up scientists to focus on experimental design, analysis, and discovery
There are also different levels of lab automation. Automation can apply to a single step in a protocol, like pipetting, or to an entire workflow that runs from start to finish with minimal human intervention.
- Task-level automation: Automating a single step, like liquid handling, sample purification, and colony picking
- Workflow automation: Automating multiple connected steps, with some manual transfers between lab machines
- Walkaway automation: Setting up an entire protocol and letting it run end-to-end without any human intervention
The more automated a workflow becomes, the more important reliability is. As labs transition from manual to automated systems, new challenges emerge alongside the benefits. If you can’t trust an automated system to recover from errors or clearly report what went wrong, automation can actually make things worse instead of better. Imagine a fast, fully automated workflow running unattended for 24 hours and quietly compromising every result along the way.
Lab automation for production vs. lab automation for R&D
One distinction that’s important to make is between automation built for production and automation built for research and development. Production automation—especially in clinical or manufacturing settings—is optimized for extremely high throughput and fixed protocols. These systems are also built around rigorous documentation and control: every item is barcoded, every handoff is logged, and every step requires sign-off. That level of lockdown is essential when you’re running the same process, the same way, every day.
Research environments operate differently. Rather than prioritizing rigidity and standardization, research requires adaptation: experiments change, protocols evolve, and iteration is constant, making production-style automation a poor fit for day-to-day R&D work. The most valuable features of research automation platforms are flexibility, modularity, and automating away setup, robot teaching, and calibration.
What does “lab automation” really mean for biologists?
For biologists, the goal of lab automation is about improving the efficiency, reliability, and capabilities within the research lab so they can perform more science and generate more data.
In practice, much of a scientist’s creativity is applied during experimental planning, while the execution phase often involves repetitive, attention-intensive tasks. These tasks—like liquid handling and plate washing—are perfect targets for automation. Automating these biological workflows shifts the role of the biologist away from constant (and error-prone) manual execution and toward experimental design, oversight, optimization, and data interpretation. Instead of spending hours on repetitive steps, scientists can focus on:
- Exploring more conditions and research variables
- Generating and analyzing richer datasets
- Leveraging insights to design better experiments
- Staying current with the literature by reading and synthesizing more scientific papers
- Communicating results through papers, talks, and collaboration
How does lab automation work?
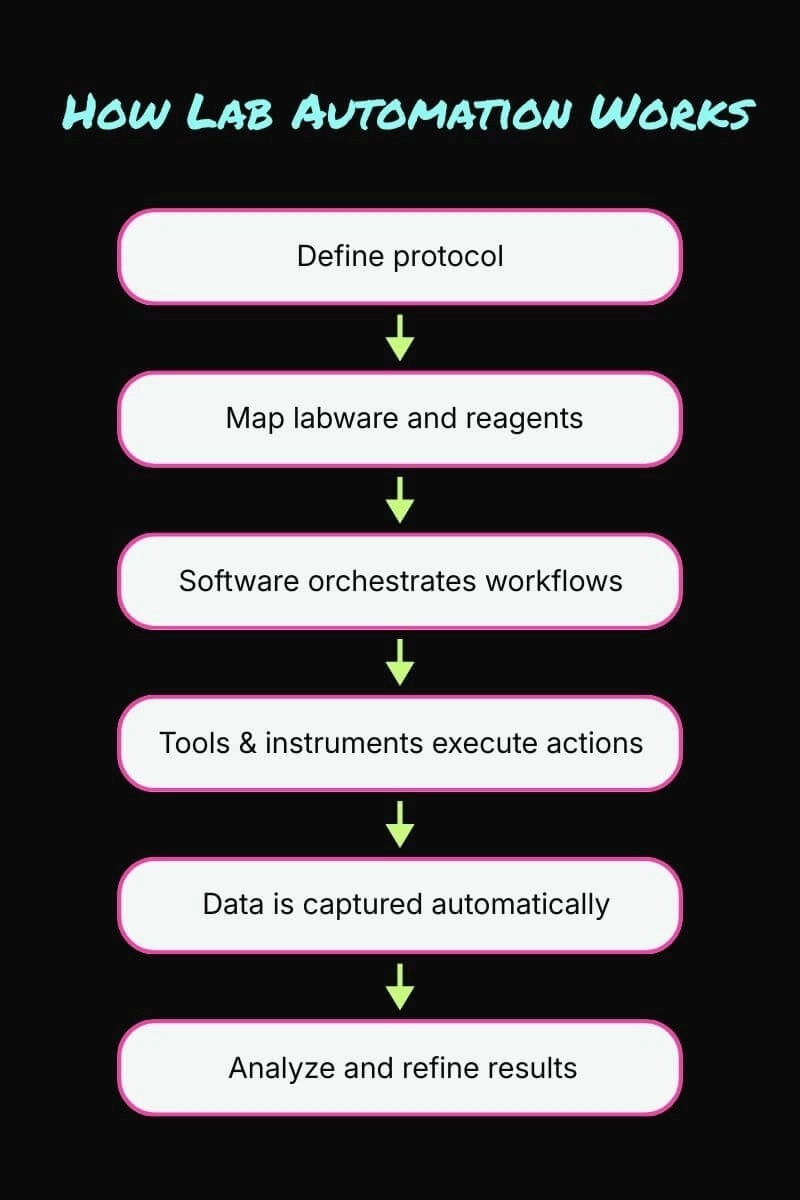
Lab automation works by coordinating hardware, software, and labware into a defined workflow. Here is a framework for creating a typical automated workflow:
- A protocol is defined based on experimental goals
- Protocol steps are mapped onto labware and reagents
- Software schedules and coordinates each step
- Tools and instruments execute physical actions
- Data is captured automatically as the experiment runs
- Results are stored and analyzed by a biologist
Again, the right automation setup depends heavily on context. Research and development labs often need flexibility and variation. Pre-clinical or production environments prioritize consistency and throughput. The same tools rarely serve both needs equally well.
The 5 Key Components of Lab Automation
In my experience, successful lab automation depends on more than just devices. It’s an ecosystem of five core components that need to work together to support laboratory workflows.
- Hardware - Hardware includes instruments like liquid handlers, incubators, heaters, coolers, shakers, imagers, and plate movers. Hardware executes the physical steps of biology.
- Software - Automation software defines what happens, when it happens, and how data is captured. This includes scheduling, protocol creation, barcoding, error handling, and reporting. It can also include information about the broader lab, such as LIMS systems, and often needs to work with other types of lab software used for analysis, data management, and collaboration.
- Plasticware and consumables - The samples have to be stored and used within consumables. Automation works well when plasticware is consistent and compatible with the hardware.
- Integration tools - Flexible systems rely on both software-level coordination and physical handoffs between machines. Many times, single-purpose robotic arms are used to connect different devices and hand off materials between them.
- Lab space and setup - Automation platforms live in real labs with real constraints. Bench space, environmental conditions, and accessibility all affect what can be accomplished in practice.
Examples of lab automation
Automation can support a wide range of lab tasks across the entire research workflow. In practice, labs tend to adopt automation in stages—starting with individual tasks and later moving toward more integrated workflows—allowing teams to build confidence, validate reliability, and adapt systems as experimental needs evolve before scaling automation further.
Early-stage lab automation
These are typically the first tasks labs automate to improve consistency and reduce manual effort:
- LIMS-based inventory tracking for samples, reagents, and plasticware
- Liquid handling and plate filling for precise, repeatable pipetting
- Plate washing for standardized cleanup steps
- Sample purification workflows
- Colony picking
- Calibrating tools and plasticware
- Plate replication to scale experiments across conditions
Late-stage lab automation
As labs mature, automation expands beyond individual tasks to support more complex, end-to-end workflows:
- Walkaway automation, where reaction setup, execution, and downstream measurement run unattended from start to finish
- Automated sample and plate movement between instruments using grippers or robotic arms
- Miniaturized workflows that enable experiments not feasible by hand
- Overnight and unattended runs that significantly increase effective lab capacity
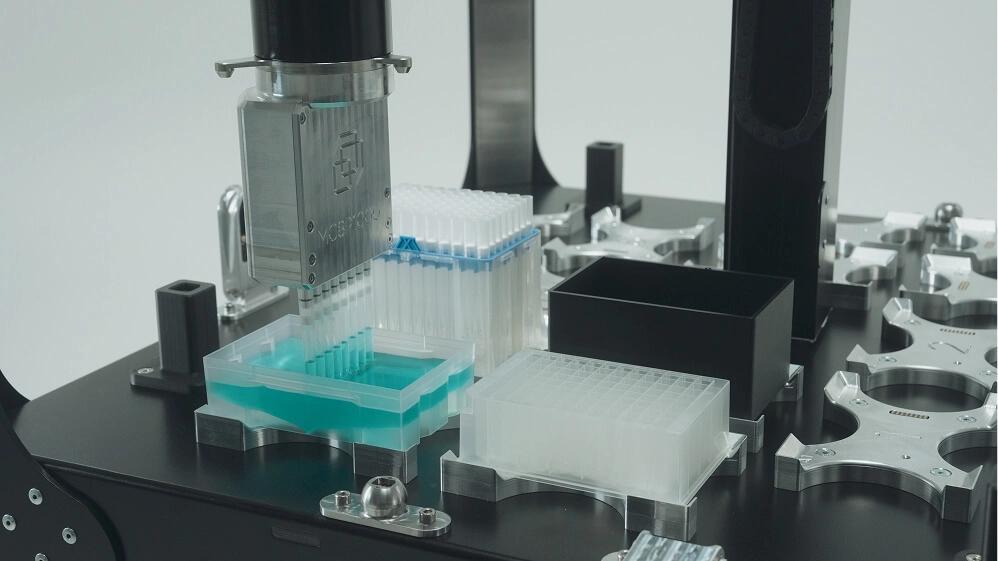
Benefits and limitations of lab automation
Lab automation can fundamentally change how experiments are executed, but its impact depends on how and where it’s applied. When implemented thoughtfully, automation increases throughput, improves data quality, and gives scientists more time to focus on discovery. When implemented poorly, it can introduce new bottlenecks or create systems that are difficult to trust or maintain. As new automation technologies resolve long-standing challenges, they also introduce new ones, and understanding that tradeoff is essential to making automation work.
The 6 benefits of lab automation
Lab automation can replace repetitive, manual tasks with more consistent, machine-executed workflows. While an experienced scientist may perform a task better by hand, those results are often difficult to reproduce across different people or long periods of time. Automation reduces day-to-day variability, improving data quality, reproducibility, and lab efficiency.
- Increased efficiency and throughput - Full automation allows labs to run more experiments in less time, including overnight and on weekends.
- Reduced errors and improved accuracy - Automated systems eliminate many common sources of human error, especially in repetitive tasks, like hit picking.
- Improved reproducibility - Standardized execution makes experiments easier to reproduce within and across labs.
- Better use of scientific time - When scientists spend less time doing repetitive, manual tasks, they can spend more time designing, analyzing, and interpreting experiments.
- Cost reduction - By reducing day-to-day variability, automation helps avoid experiment remediation, eliminating the time, reagent costs, and delays associated with repeating or adapting experiments.
- Richer data collection - Automated workflows capture structured data and metadata by default, often accessible through APIs, without adding extra work.
The 4 challenges of lab automation (and how to mitigate them)
All new solutions address old challenges while introducing new ones that must be mitigated. Despite its advantages, I’ve also found that not all lab automation is a plug-and-play solution. New challenges arise not from the technology itself, but from how it integrates into existing lab workflows, teams, and infrastructure.
- Integration complexity - Not all instruments and software work well together. Choosing flexible platforms and open systems reduces friction.
- Training and adoption - Automation changes how people work. Successful automation projects include training and platform adoption.
- Cost - Automation systems can range from tens of thousands to hundreds of thousands of dollars. Finding the right platform means finding the right match for your needs and budget
- Change management - Automation challenges the status quo. Clear goals and early wins help build trust.
Lab automation solutions, tools, and technologies in 2026
In 2026, lab automation is becoming more modular, more intelligent, and more adaptable. As biology grows more exploratory and data-driven, automation systems are being asked to do more than execute fixed protocols. They need to support variation, iteration, and change.
At Trilobio, we’re seeing several clear shifts across the field:
- Smaller and more collaborative robots
- AI-enhanced systems that optimize workflows
- Tighter integration between automation and LIMS
- Flexible platforms built for the specific requirements of R&D
- Specialized instruments for DNA synthesis, driving down costs and expanding access to custom genetic constructs
This shift toward flexible, research-first automation is critical as biology becomes more exploratory and data-driven. At Trilobio, we’re building powerful, affordable lab automation that is biologically aware and designed for R&D from the ground up. Our platform is built to support real biological workflows and is designed by biologists, for biologists.
The labs that succeed with automation over the next decade will be the ones that choose systems designed to adapt as the biology changes.
Sign up for the latest insights and updates.
By providing this information, you agree to be kept informed about Trilobio’s products and services.